Rabbits, guinea pigs, rats, and other small animals are popular pets and daily patients in our small animal practices. As in all animals, mycoplasmas are also of great importance in small mammals. They are usually host-specific, so that different mycoplasma species have clinical relevance for (almost) every animal species.
Mycoplasma ‒ a special bacteria
Mycoplasmas are gram-negative bacteria. They are facultative aerobes and obligate parasites. They are unique mainly because of the absence of a cell wall – they are only bounded by a cytoplasmic membrane. They are also the smallest self-replicating organisms. They prefer the epithelia of the respiratory and urogenital tracts, articular cartilage, eyes, and mammary glands. Mycoplasma infections are chronic.
Due to their morphological peculiarities, mycoplasmas can only be cultured under very special conditions. This is a lengthy and expensive process that is not relevant for routine diagnostics. Serology can only be used for a few mycoplasma species, but since seroconversion can take months and does not provide any information about clinical relevance, it is not a suitable diagnostic tool in cases of illness. The rapid and reliable detection of mycoplasma infections is possible using the polymerase chain reaction (PCR).
Thirteen of the currently 159 classified species of the genus Mycoplasma are associated with small mammals (as of 2022, see Table 1). Of these 13 species, only a few are truly clinically relevant, and we will take a closer look at them below.
Table 1: Classified Mycoplasma species in small mammals – M. pulmonis and M. cavie are of particular clinical relevance (Image source: Klas, E-M., Liebscher)
| Pathogen | Disease | Species |
|---|---|---|
| Mycoplasma pulmonis | murine respiratory mycoplasmosis (MRM), genital tract infections, arthritis | Mouse, Rat, Rabbit (Guinea Pig, Syrian Hamster, Human) |
| Mycoplasma arthritidis | mostly subclinical, rarely arthritis | Mouse, Rat |
| Mycoplasma neurolyticum | “rolling mouse syndrome,” only experimental, no known natural infections | Mouse, Rat |
| Mycoplasma collis | conjunctivitis (one report from 1980) | Mouse, Rat |
| Mycoplasma muris | none known | Mouse |
| Mycoplasma coccoides | mostly subclinical, rarely hemolytic anemia | Mouse |
| Candidatus Mycoplasma haemomurismusculi | mostly subclinical, rarely hemolytic anemia | Mouse (Wild rodents) |
| Candidatus Mycoplasma haemomuris ssp. ratti | mostly subclinical, rarely hemolytic anemia | Rat (Wild rodents) |
| Candidatus Mycoplasma ravipulmonis | grey lung disease | Laboratory Mice |
| Mycoplasma caviae | respiratory diseases | Guinea Pig |
| Mycoplasma cavipharyngis | none known | Guinea Pig |
| Mycoplasma cricetulli | none known | Chinese Hamster |
| Mycoplasma oxeniensis | none known | Chinese Hamster |
Mycoplasma pulmonis
Mycoplasma (M.) pulmonis is the causative agent of murine respiratory mycoplasmosis and is considered the most important pathogen in respiratory diseases of rats and mice. The signs of disease range from non-specific symptoms, such as reduced general well-being and weight loss, to rhinitis, otitis, dyspnoea, and severe pneumonia. In addition, arthritis and fertility disorders such as endometritis and salpingitis occur in connection with M. pulmonis infections (especially in rats).
M. pulmonis has also been detected in guinea pigs, rabbits, and hamsters. However, a connection with respiratory symptoms has only been established in rabbits; so far, this has been more common in food-supplying animals than in pets. At the moment, it is assumed that M. pulmonis does not cause disease in humans, although it has been detected (serologically and directly by PCR) in humans. In the differential diagnosis of disease in rats, Streptococcus pneumoniae, Corynebacterium kutscheri, and Filobacterium rodentium (CAR bacillus) are of particular importance.

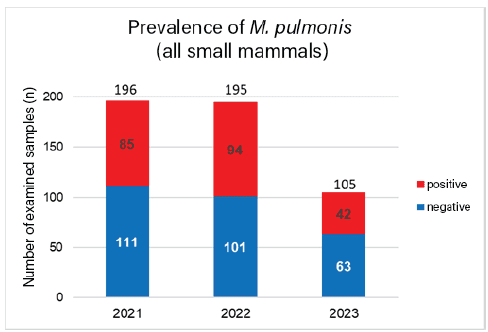
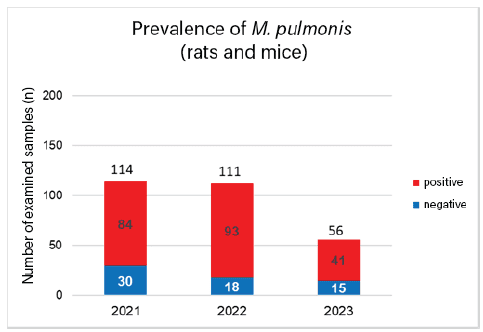
We evaluated 496 M. pulmonis PCR tests from submissions between 2021 and 2023. Of these 496 tests, 221 were positive, which corresponds to 44.6%. Looking at the individual years, 43.4% (85/196) were positive in 2021, while M. pulmonis was detected in 48.2% (94/195) in 2022 and 40% (42/105) in 2023 (Fig. 1). PCR tests were carried out on many different small mammals, including rats, mice, guinea pigs, rabbits, gerbils, hamsters, and other species. Since the pathogen mainly plays a role in mice and rats, we looked at these species separately (Fig. 2). Here, a total of 77.6% (218/281) of the submissions were positive. This is consistent with the literature, which reports a prevalence of 70% in pet rats (PCR detection). M. pulmonis should always be considered in the differential diagnosis of rats and mice with respiratory symptoms.
Mycoplasma caviae
This pathogen has only recently been the subject of increased attention, although it was first described in the 1900s. M. caviae is responsible for symptoms in guinea pigs that are similar to those of M. pulmonis in mice and rats: anorexia, lethargy, rhinitis, dyspnoea, and severe interstitial pneumonia. Here, too, arthritis and fertility disorders (metritis) are possible. In addition, conjunctivitis and lymphadenitis have been described. Differential diagnoses are mainly infections with Streptococcus pneumoniae and Bordetella bronchiseptica.
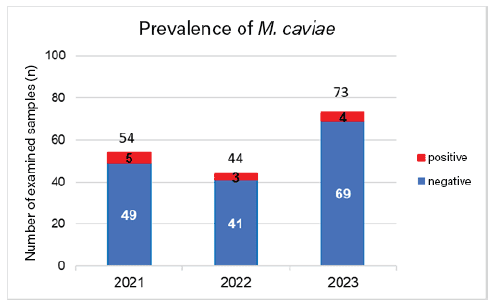
We retrospectively examined a total of 171 samples (nasal and/or throat swabs, rinses) from guinea pigs from 2021 to 2023. M. caviae was detected in 9.3% (5/54) of the samples in 2021, in 6.8% (3/44) in 2022, and in 5.5% (4/73) of the samples in 2023 (Fig. 3). Overall, 7% (12/171) of the guinea pigs examined were M. caviae positive. Since these investigations were carried out without any specific mandate and without any prior knowledge of the respective guinea pigs, the actual prevalence is presumably higher. The only data available from the literature dates from 1971, when the prevalence was 10%.
Mycoplasma spp.
To date, no mycoplasma species has been described in rabbits, but Mycoplasma spp. have been detected in rabbits with respiratory symptoms. They may be involved in the so-called “rabbit rhinitis complex.” Here, too, the symptoms range from weight loss, apathy, nasal discharge, and dyspnoea to pneumonia. Furthermore, mycoplasmas have been detected in conjunction with conjunctivitis, so the detection of Mycoplasma spp. is particularly useful in rabbits with respiratory or eye problems. Differential diagnoses would include, in particular, pathogens from the Pasteurellaceae or Enterobacteriaceae family and Pseudomonas spp.
Conclusion
In particular, in small mammals with respiratory symptoms, a mycoplasma infection is a possible differential diagnosis; this applies especially to rats and mice. Mycoplasma infections are factor diseases, and mycoplasma detection is only necessary in conjunction with clinical symptoms. PCR is the detection method of choice (from smears without transport medium or rinsing samples).
Dr Eva-Maria Klas
FURTHER LITERATURE
- Klas E-M, Liebscher J. Atemwegsassoziierte Mykoplasmeninfektionen beim Kleinsäuger. Kleintier konkret 2024; 27 (S 01): 12-26. DOI: 10.1055/a-2241-4125
- Klas E-M, Kaiser E-M, Scherzer J, Kerner K, Müller E. Etablierung einer PCR zum Nachweis von Mycoplasma caviae und die Nachweishäufigkeit beim Meerschweinchen, Pos-ter präsentiert auf der 32. Jahrestagung der DVG-Fachgruppe Innlab; 2024 Feb 02-03; Hannover, Deutschland
- Balzey, B., Mykoplasmenpneumonie des Meerschweinchens Ein Bericht aus unserem Laboralltag https://www.ua-bw.de/pub/beitrag.asp?subid=1&Thema_ID=8&ID=2552 (05.09.2017)
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 1998; 62: 1094–1156. DOI: 10.1128/mmbr.62.4.1094-1156.1998

