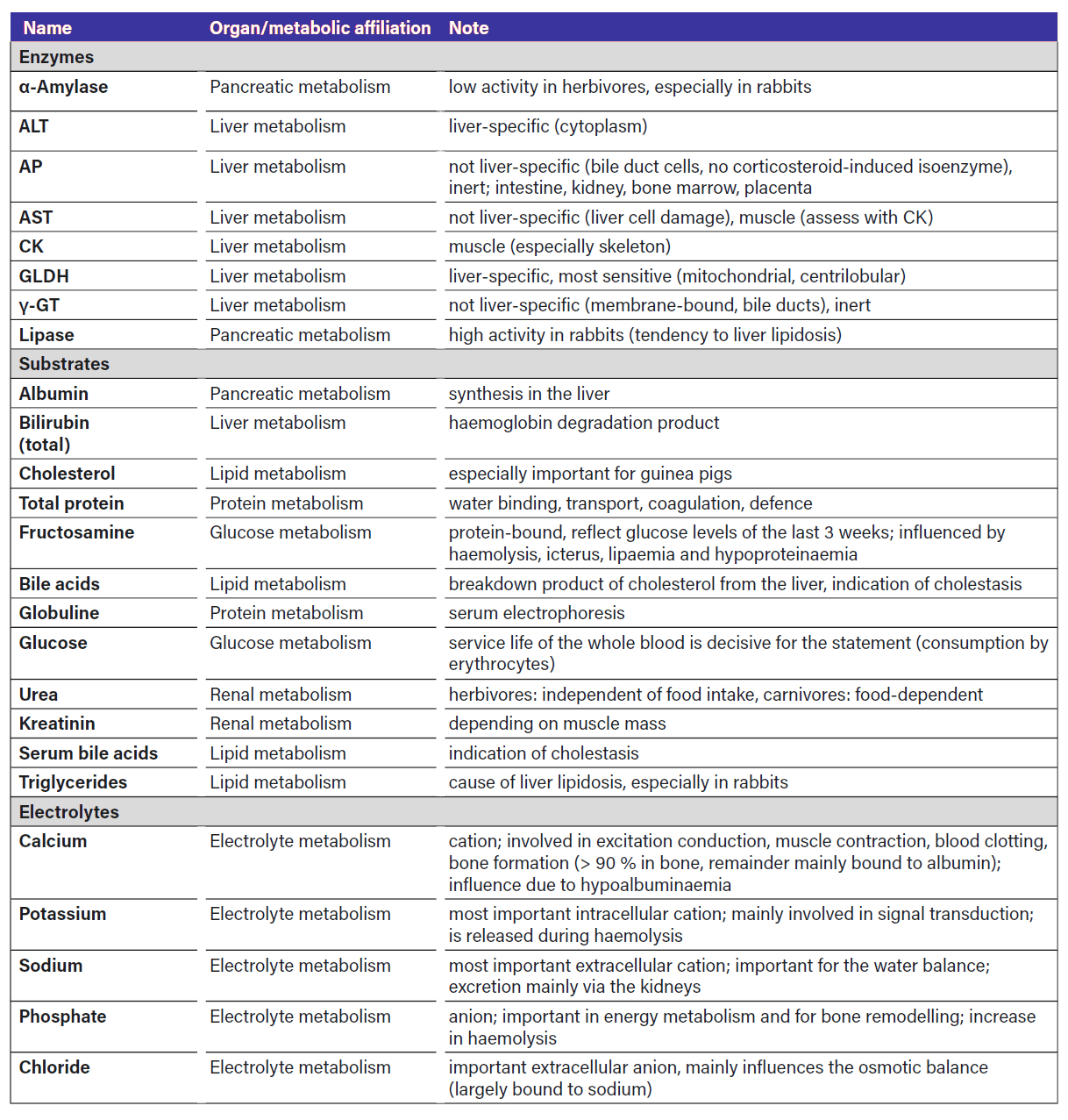
Small mammals are fleeing and prey animals. Serious illnesses might remain unrecognised for a long time, otherwise the animals can easily become victims of predators! Laboratory diagnostics are therefore an important part of the diagnostic process, especially in the case of non-specific symptoms such as apathy, inappetence and lack of mobility. Not only changes in the red (anaemia, dehydration) and white blood count (pseudo-left shift, lymphoma) can be indicative. Changes in the activity and concentration of clinical-chemical parameters (enzymes, substrates, electrolytes) show which organs are affected or what type of metabolic change is present. The most important parameters are listed in Table 1 for “quick reference”.
Liver metabolism
The enzymes GLDH, ALT, AST, γ-GT, AP and the substrates glucose, albumin, urea, bilirubin, triglycerides and serum bile acids are also considered liver parameters in small mammals. These are determined in either serum or plasma.
The GLDH (glutamate dehydrogenase) is found in the mitochondria of liver cells (centrilobular) as well as heart and kidney cells (Wesche 2014). It is the most sensitive liver enzyme and responds even to mild cell damage (acute hepatopathies due to anorexia (fat storage) and/or intoxication) and is a good marker for acute, incipient liver problems (Leban-Danzl et al. 2016). The measurement is not yet available for in-house diagnostics.
The ALT (alanine aminotransferase) is considered to be liver-specific and is mainly found in the cytoplasm of liver and heart muscle cells (Hein 2014). It is less sensitive than GLDH, is only released as liver cell damage progresses and therefore indicates more severe and/or chronic liver damage (Leban-Danzl et al. 2016). A correlation between liver cell death and ALT activity has been described (Jenkins 2000).
The AST (aspartate aminotransferase) is similarly sensitive as the ALT, but is not specific to the liver, as it also occurs in the heart and skeletal muscle. An increase in AST activity should therefore always be interpreted together with CK activity (muscle enzyme) and the other liver parameters in order to differentiate between a muscle-associated increase in concentration (Leban-Danzl et al. 2016). ALT and AST are also present in certain quantities in erythrocytes, i.e. they are also released in small quantities during haemolysis without a liver problem being present.
AP (alkaline phosphatase) and γ-GT (gamma-glutamyltransferase) are mainly found in the bile ducts, but are also not liver-specific and are rather inert (Hein 2014). If their activity is increased and the bilirubin and serum bile acid (SGS) concentration in the serum/plasma also rises, one can assume cholestasis. In rabbits, AP is particularly inert and a steroid-sensitive isoenzyme does not develop in them. In other small mammals, AP activity is increased, especially in young animals, due to increased bone metabolism and possibly also in other hepatopathies, osteopathies, pregnancy and bone loss (Leban-Danzl et al. 2016).
Liver metabolism is usefully assessed in conjunction with the substrates glucose, albumin, urea, bilirubin, triglycerides and bile acids in order to differentiate between pre- and intrahepatic causes of cholestasis (posthepatic). Rabbits have a particularly active fat metabolism. In phases of anorexia, there is rapid fat mobilisation and storage in the liver (hepatic lipidosis) (Hein 2014). In liver cirrhosis, an increase in enzymes is often no longer detected as the capacity of the liver cells is exhausted. Blood parasites or autoimmune haemolytic anaemia have not yet been described in small mammals (Hein 2019).
Accordingly, mild hepatopathies only begin with an increase in GLDH activity. Depending on the severity and duration of the problem, AST and ALT activity increase and all other liver parameters only change in the event of severe damage. Massive changes in liver parameters occur primarily in RHD, massive liver coccidiosis, intoxications and/or liver lobe torsions.
Renal metabolism
Urea and creatinine concentrations are considered reliable kidney parameters in small mammals. The data available on SDMA
measurement in small mammals is currently still too limited.
In carnivorous or insectivorous small mammals, the urea concentration is, as in dogs and cats, dependent on food intake (protein-rich food) (Hein 2014).Herbivorous small mammals only consume a small amount of protein with their food. Therefore, in contrast to carnivorous or insectivorous small mammals the urea concentration in their blood is independent of their diet. An isolated increase in the urea concentration can be an indication of gastrointestinal haemorrhage (reabsorption of blood) (Hein 2014). Late-stage liver insufficiency and/or a reduced protein intake can lead to a drop in urea concentrations.
Creatinine is a non-specific muscle parameter and, as the end product of endogenous muscle metabolism, is a reliable and nutrition-independent renal parameter. The creatinine concentration is influenced accordingly by muscle mass, exercise activity and renal function (Hein 2014).
If urea and creatinine concentrations are elevated at the same time, this is referred to as azotemia. Azotaemia can occur prerenally (dehydration, hypovolaemia, renal hypoperfusion), renally (acute or chronic renal insufficiency) and/or postrenally (obstruction of the urinary tract).
Pancreatic metabolism
Although only of minor importance in herbivores due to the predominance of bacterial digestion, the measurement of α-amylase and lipase activity is also possible in small mammals. Rabbits have a high lipase activity, which explains the rapid mobilisation of fat and their tendency to liver lipidosis during periods of starvation, while the α-amylase activity is rather low. In contrast to others, rabbits tend to have permanently high glucose and fructosamine concentrations.
Glucose metabolism
Herbivorous small mammals are never physiologically fasting.
The glucose and fructosamine concentrations provide information on the sugar metabolism. Fructosamines are glycosylated serum proteins. The glucose concentration of the last 3 weeks is reflected in the fructosamine concentration. Longer-term changes in the glucose concentration (diabetes, insulinoma) can therefore be detected more easily, while short-term changes (e.g. short hunger phases, glucose administration) have hardly any influence.
Both substrates are determined from promptly centrifuged, separated serum or heparin plasma, regardless of the time of the last feed intake (except ferrets 2-4 hours off food). Otherwise there will be falsely low glucose concentrations due to degradation by the remaining erythrocytes and changes in the fructosamine concentration due to haemolysis. Hypoproteinaemia of any kind also leads to falsely low fructosamine concentrations, and lipaemia also influences the fructosamine concentration. The results should therefore always be critically reviewed and checked if necessary.
Diabetes mellitus is rather rare in small mammals and is mostly diet-related. Differential diagnoses for hyperglycaemia are: stress, iatrogenic intake, hormone influence (glucocorticoids, progesterone) (rather mild) and in rabbits ileus (severe). In rabbits, the glucose and fructosamine concentration is permanently high, as they only metabolize carbohydrates slowly, but quickly switch to gluconeogenesis (Harcourt-Brown and Harcourt-Brown 2012). Determining the glucose concentration can therefore be helpful in rabbits with suspected ileus. The higher the glucose concentration, the more likely an ileus is in an
inappetent rabbit, and the longer it persists, the worse the prognosis (Harcourt-Brown and Harcourt-Brown 2012). If hyperglycaemia and hyponatraemia (sodium < 129 mmol/l) occur together in severely ill rabbits, the mortality rate is 2.3 times higher (Bonvehi et al. 2014).
Lipid metabolism
Fat metabolism includes triglycerides, cholesterol and serum bile acids. Increases in triglyceride concentrations occur rapidly in rabbits during periods of anorexia and are the first indication of impending liver lipidosis. Hypercholesterolaemia is mainly described in guinea pigs and is associated with fatty infiltrations in the liver and other tissues (Hein 2014).
Protein metabolism
The total protein and albumin concentration can be measured photometrically and the albumin and globulin fractions by means of electrophoresis. These measurements are important for symptoms associated with a disturbance in the water and
protein balance, such as diarrhoea, polydipsia/ polyuria, weight loss, etc. Acute phase proteins have so far only played a minor role in small mammals.
Electrolyte metabolism
Sodium, potassium, calcium and phosphate concentrations are also measured in small mammals. The determination of other electrolytes such as chloride, magnesium, iron etc. is also possible.
Herbivorous small mammals do not take up calcium according to their needs, but according to their diet and – except in the case of calcium deficiency – also independently of vitamin D via intestinal absorption. This results in physiological fluctuations in the serum calcium level. In rabbits, guinea pigs and degus, up to 65 % of excess calcium is excreted via the urinary tract (in contrast to < 2 % in most other pets) (Hein 2014). Excess calcium results in urinary gravel and uroliths in the urinary tract. Chinchillas largely excrete excess calcium in their faeces. Uroliths are therefore rare in them, but tissue and possibly aortic calcifications are all the more common. Meaningful studies on the relationship between calcium and phosphate concentrations in small mammals are still lacking.
Potassium plays a similar role in small mammals as in dogs and cats, but their tolerance to fluctuations in potassium concentration, e.g. in neoplasia, appears to be greater. Hyperkalaemia occurs during whole blood transfusions as a result of haemolysis (release from thrombocytes, leucocytes and erythrocytes).
Conclusion
With the help of clinical-chemical parameters, the metabolic situation in small mammals can be assessed well and diseases can be diagnosed quickly and easily.
Jana Liebscher
Dr. Jutta Hein
FURTHER LITERATURE
- Bonvehi C, Ardiaca M, Barrera S, Cuesta M, Montesinos A. Prevalence and types of hyponatraemia, its relationship with hyperglycaemia and mortality in ill pet rabbits. Vet Rec 2014; 174(22): 554. doi:10.1136/vr.102054
- Harcourt-Brown FM, Harcourt-Brown SF. Clinical value of blood glucose measurement in pet rabbits. Vet Rec 2012; 170(26): 674. doi:10.1136/vr.100321
- Hein J. Labordiagnostik bei Kaninchen, Meerschweinchen, Chinchilla und Frettchen. In: Moritz A, Hrsg. Klinische Labordiagnostik in der Tiermedizin. 7. Aufl. Stuttgart: Schattauer; 2014: 784-803.
- Hein J. Labordiagnostik bei Kleinsäugern: Präanalytik und tierartspezifische Befundung. Hannover: Schlütersche Verlagsgesellschaft; 2019.
- Leban-Danzl A, Hartmann K, Majzoub-Altwecker M, Hermanns W, Sauter-Louis C, Hein J. Sensitivity of liver parameters in diagnosing liver diseases in rabbits. Berl Munch Tierarztl Wochenschr 2016; 11/12(129): 518-26.
- Jenkins JR. Rabbit and ferret liver and gastronintestinal testing. In: Fudge AM, ed. Laboratory medicine. Avian and exotic pets. Philadelphia: Saunders; 2000: 291-304.
- Wesche P. Clinical pathology. In: Meredith A, Lord B, eds. BSAVA Manual of Rabbit Medicine. Gloucester: British Small Animal Veterinary Association; 2014: 125-37.