
Image source: envatoelements
Dermatophytes are ubiquitous, keratinophilic, filamentous fungi that infect skin, hair, and claws. They are a serious concern due to their zoonotic potential (de Matos and Kalivoda 2013). According to the National Center for Biotechnology Information (NCBI, Bethesda, Maryland, USA), they are currently classified into 9 genera (including Microsporum, Trichophyton and Arthroderma) (Schoch et al. 2020). Microsporum spp. and Trichophyton spp. are the main causes of dermatophytoses in animals, especially in dogs and cats (Paryuni et al. 2020). In small mammals, skin fungi from the Trichophyton benhamiae complex (several anthropophilic and zoophilic species), predominantly Trichophyton (T.) benhamiae, rarely Microsporum (M.) canis or other species (Table 1), cause the so-called ringworm (Fréalle et al. 2007, ESCAPP 2021).
The correct classification is complicated as originally, T. benhamiae was classified according to morphological criteria such as growth behaviour, phenotype (yellow/white) and microscopic appearance, and the anamorphic (asexual) growth form was initially named T. mentagrophytes (Fréalle et al. 2007). DNA sequencing has since differentiated the main teleomorph (sexual) form, resulting in them being re classified as Arthroderma (A.) benhamiae (Fréalle et al. 2007). The current designation since 2017 is T. benhamiae complex (Hoog et al. 2017). In older publications one finds the name T. mentagrophytes, in newer ones T. benhamiae – but both refer to the same species.
WHO CAN GET IT?
Guinea pigs and hedgehogs are most frequently infected with dermatophytes, while other small mammals are rarely infected (Table 1).
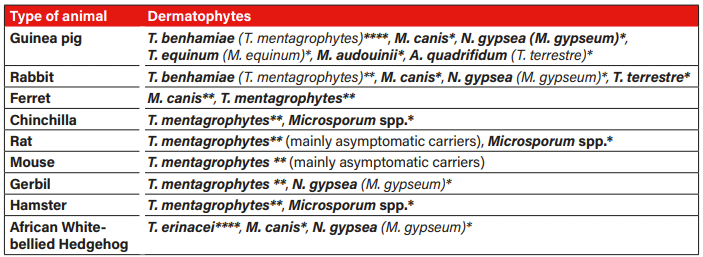
Tab. 1: Occurrence and frequency of dermatophytes in small mammals: **** frequent, ** rare, * very rare; T.=Trichophyton, M.=Microsporum, N.=Nannizzia, A=Arthroderma; old designation depending on time of publication; Sources: Berlin et al. 2020, de Matos and Kalivoda 2013, ESCCAP 2021, Kraemer et al. 2012, Overgaauw et al. 2017, Pignon and Mayer 2011, Vangeel et al. 2000.
The occurrence of T. benhamiae in guinea pigs varies depending on the type of environment in which they are kept. In a study involving 59 guinea pigs from 15 pet shops in Berlin, 90 % (53/59) tested positive through PCR (Kupsch et al. 2017). In the Netherlands, the prevalence of T. mentagrophytes in pet shops was 16.8 % (30/179) in guinea pigs and 3.8 % (8/213) in rabbits (Overgaauw et al. 2017). In another recent German study in guinea pigs, both breeding and pet guinea pigs were examined (Berlin et al. 2020). From a total of 21 private breeding herds, 68.8 % (262/381) of the animals tested positive for dermatophytes – 55.4 % of them for T. benhamiae, 13.4 % for others (7.1 % T. interdigitale, 6.0 % T. rubrum, 2.6 % T. erinacei, 2.3 % T. verrucosum, 1.3 % T. mentagrophytes). 92.7 % of all animals were asymptomatic carriers. There was no predisposition to gender. Longhaired breeds with curls (74.0 %) and Rex guinea pigs (68.1 %) were more often affected than shorthaired breeds, animals kept indoors (67.2 %) were more often than animals kept outdoors. Catteries with frequent changes in the animal population (76.0 %) were significantly more infected with T. benhamiae than animals from self-sufficient catteries (37.1 %) (Berlin et al. 2020). Although it is often described that dermatophytes are more prevalent in young animals (de Matos and Kalivoda 2013, ESCCAP 2021, Kraemer et al. 2012), this could not be confirmed in guinea pigs from breeding herds in this study (Berlin et al. 2020).
In the evaluation of 9636 laboratory examinations of pet guinea pigs, the prevalence of dermatophytes was 3.9 % (382/9636), and 36.9 % (382/1035) in animals with clinical suspicion. T. benhamiae was detected in 98.2 % of animals with clinical suspicion (Berlin et al. 2020).
Another German study from 2012 also investigated the prevalence of dermatophytes in laboratory submissions of pet guinea pigs by means of cultural examination (Kraemer et al. 2012). Dermatophytes were detected in 38.1 % (431/1132) of pet guinea pigs, of which T. mentagrophytes was detected in 91.6 % (395/431). In addition, healthy guinea pigs were sampled: 8.5 % (14/164) were asymptomatic carriers (Kraemer et al. 2012). The prevalences in pet guinea pigs have thus remained approximately the same.
Pet rabbits, on the other hand, showed dermatophytes in only 8.1 % (83/1021) in the laboratory sample evaluation of 2012, and T. mentagrophytes in 72.3 % of these (60/83). There were no asymptomatic carriers in healthy rabbits in this study (0/140) (Kraemer et al. 2012). In a Belgian study, 3.8 % (4/104) of pet, breeding and laboratory rabbits were asymptomatic carriers (Vangeel et al. 2000). In the German study, rabbits with positive fungal cultures were younger than animals with negative fungal cultures or healthy animals (Kraemer et al. 2012).
In hedgehogs, dermatophytoses are frequently caused by T. erinacei, very rarely by M. canis and Nannizzia (N.) gypsea (M. gypseum) (Pignon and Mayer 2011). In animals displaying clinical signs, T. erinacei could be diagnosed in 4 out of 5 animals. Asymptomatic carriers have been described in 11.5 % (47/408) (Pignon and Mayer 2011).
Dermatophytes are common in guinea pigs and rare in rabbits. Guinea pigs from pet shops (up to 90 %) and private breeding stock (68.8 %) are significantly more affected than pet guinea pigs (up to 38.1 %). The prevalence in asymptomatic guinea pigs varies between 3.9 % (pet animals) and 92.7 % (breeding stock) depending on the husbandry.
WHO TRANSMITS IT?
Dermatophytoses are zoonoses! Transmissions to humans are frequently described. During the corona pandemic, the number of human, T. benhamiae infections increased in line with the increase of keeping small mammals during the pandemic period (Uhrlaß et al. 2023). Children and adolescents are predominantly affected, which is due the often close contact with pets. Infection can be dangerous for immunocompromised people (National Research Platform for Zoonoses 2020).
Guinea pigs are the most common source of human infections (Berlin et al. 2020, Kupsch et al. 2017, National Research Platform for Zoonoses 2020, Nenoff et al. 2014, Uhrlaß et al. 2023). However, rabbits can also become potential sources of infection. In 27.3% (3/11) of affected households with dermatophyte-positive pet rabbits, the owners, in this case mainly the children, were infected (Krämer et al. 2012). Transmission of dermatophytes from European hedgehogs and African white-bellied hedgehogs to humans also occurs (Pignon and Mayer 2011, Riley and Chomel 2005).
Transmission of dermatophytes occurs both directly and indirectly via spore-contaminated objects, bedding, brushes and combs (de Matos and Kalivoda 2013, ESCCAP 2021). Infected ferrets often live together with cats (de Matos and Kalivoda 2013). Hedgehogs probably become infected through direct contact at the time of mothering or during fights and courtship behaviour, as the clinical signs are often visible on the head often occur on the head (Pignon and Mayer 2011). High numbers of animals in a confined space increase the pressure of infection (Berlin et al. 2020, de Matos and Kalivoda 2013).
Clinical manifestation often occurs during immunosuppression, stress, other underlying diseases, parasite infestation, weaning in young animals and warm, humid climates. Injuries, cracks in the skin and increased skin moisture are conducive to infection (Berlin et al. 2020).
Typical clinical symptoms are circular alopecia, breakage of hair, dandruff, erythema, yellow crusts, and sometimes pruritus (de Matos and Kalivoda 2013). Initially, dermatophytoses often begins on the head (bridge of the nose, eyelids and pinnae) (Figure 1). Secondary pyoderma often develops and spreads to the (front) paws, claw bed and later to the whole body (de Matos and Kalivoda 2013). Hedgehogs can present with non-pruritic, scaly lesions, especially on the head, and loss of spines (de Matos and Kalivoda 2013, Pignon and Mayer 2011).

Fig. 1: Young guinea pig with typical crusted lesion on the bridge of the nose in dermatophytosis.
HOW TO DIAGNOSE?
Samples for mycological diagnosis are best taken using the McKenzie hairbrush technique (Figure 2). To increase the sensitivity, it is best not only to brush the affected skin areas, but the entire animal, for 1 – 2 minutes with a sterile brush (toothbrush, cytobrush) (Berlin and Gräser 2020). In addition, some hairs including the root should be taken from the transition of the altered skin areas and sent in a sealable bag or container. By means of microscopic examination (e.g. trichogram), fungal hyphae or spores can be detected microscopically on and/or in the hair. However, a negative result does not exclude an infection. A woods lamp can only detected infections with M. canis. However, fluorescence is only seen in about 50 % of infections. Fluorescence is caused by metabolites deposited in the hair follicles that are formed during an infection and can usually be detected 7 – 14 days after infection (Moriello et al. 2017).
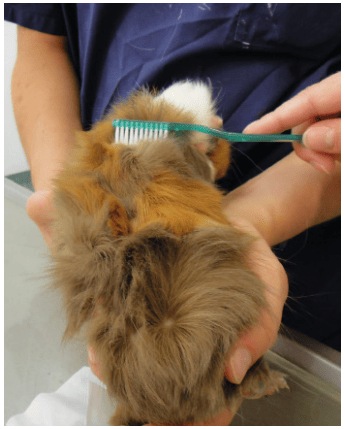
Fig. 2: Sample collection for dermatophyte examination using a McKenzie brush.
False positive results can be caused by lint, topical medications, or even soap residues (Moriello et al. 2017).
Detection by means of mycological culture is carried out by cultivation on special culture media with subsequent macroscopic and microscopic differentiation. To avoid overgrowth of moulds, it is advisable to wipe the coat with an alcohol-dampened cloth before taking the sample. In addition, selective culture media are used in the laboratory to suppress the growth of contaminants. The duration of the mycological culture varies; positive findings can often be available within 1 week. In negative cases, the culture is usually incubated for 3 – 4 weeks to rule out growth.
The national consultant laboratory for dermatophytes recommends detection by PCR due to the faster analysis time of only 2 – 3 days. Differentiation of the species is possible. Since PCR also detects dead spores, therapy control by PCR is only useful after thorough removal of the spores by bath therapy and should be considered when interpreting the results. Therefore, fungal culture is recommended for therapy control (ESCCAP 2009). The histological examination of a skin biopsy by means of special staining also provides a result within a few days. However, it is only conclusive in positive cases, and is invasive – therefore not the diagnostic method of choice (Weider 2015). Which procedure is most effective depends on the clinic, the sample collection and the study. Recent studies showed a high sensitivity of microscopy followed by PCR for visible dermatophyte lesions (Gnat et al. 2022). In asymptomatic animals, PCR is usually superior (Berlin and Gräser 2020, Nikaein et al. 2023). However, the most recent literature recommends combining PCR and culture for a reliable result (Frost et al. 2022).
TREATMENT
The treatment of dermatophytoses should be carried out according to current ESCCAP recommendations (combination of systemic [itraconazole] and bath therapy [enilconazole 2 × weekly]) (Berlin and Gräser 2020, ESCCAP 2009, Hein 2016, Körnig 2021). The solution should not be rinsed out. To protect against hypothermia, animals should be properly wrapped in a towel after the wash treatment.
Local treatment of affected skin areas alone should be strictly rejected because of the high zoonotic risk, which also emanates from asymptomatic animals (Berlin and Gräser 2020, ESCCAP 2009, Hein 2016, Körnig 2021).
All animals in the herd are to be treated regardless of the clinical picture. Optimally, the therapy ends after 2 negative controls. Environmental treatment (cage, house, care utensils, clothing, etc.) is mandatory alongside the bathing treatments. Cage equipment that is difficult to disinfect should be replaced with cardboard boxes etc. for the duration of the therapy.
Testing before introducing new animals into the herd is indicated, especially for guinea pigs that are often subclinically infected (ESCCAP 2009).
CONCLUSION
Dermatophytes can occur in almost all small mammals, but are most common in guinea pigs and hedgehogs. As asymptomatic carriers guinea pigs are the main source of human infections, especially in children, testing should be carried out on new animals that are being introduced to the herd. Self-protection is important and must not be neglected.
Jana Liebscher, Dr Jutta Hein


